Luke Lab
Research Publications Group Members BiographyRNA-DNA hybrids, telomere biology & genomic instability

Linear chromosome ends are safeguarded by telomeres. Telomeres shorten during each cell division and eventually replicative senescence occurs as a result of DNA damage checkpoint activation. Replicative senescence acts as a tumour suppressor, but can also contribute to aging. Cancer cells must find a means of overcoming the checkpoint barrier and furthermore re-elongate their telomeres in order to achieve immortality. We are interested in understanding how cells transition through replicative senescence when short/dysfunctional telomeres arise and checkpoints become activated. We are also investigating how some cells are able to override the checkpoint signal, even though DNA damage persists. We continue to study TERRA, a non-coding telomeric RNA that forms RNA-DNA hybrids at telomeres. More recently we have expanded our interest to other forms of RNA/DNA hybrids. Indeed, RNA/DNA hybrids are either formed through transcription, as long stretches in cis and in trans, or during DNA replication when single ribonucleotides are mistakenly incorporated into the DNA helix. At first RNA-DNA hybrids were seen solely as a form of DNA damage, but it has become evident that RNA-DNA hybrids also have beneficial roles. Therefore the regulation of these structures is crucial and intricate.
We are currently focused on:
TERRA (a non-coding TElomere Repeat containing RNA)
We investigate TERRA regulation during the cell cycle and senescence to understand how its regulation and deregulation affect telomere homeostasis and genomic instability. Human ALT (alternative telomere lengthening) tumours maintain their telomeres by homologous recombination and are usually associated with poor prognosis.
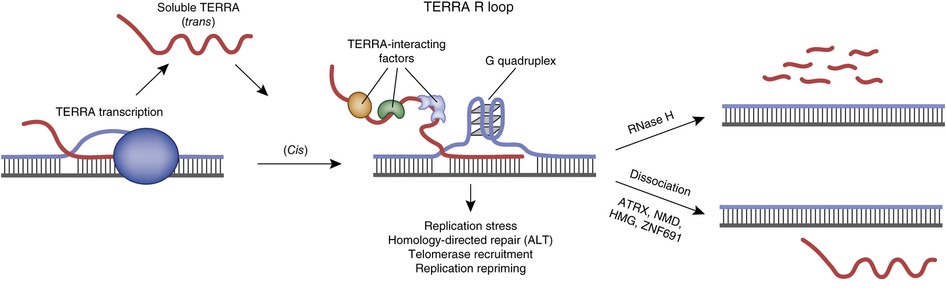
Novel factors regulating RNA-DNA hybrids
We are keen on characterising novel factors important for maintaining a stable genome when both long RNA-DNA hybrids and single ribonucleotide insertions accumulate. Factors involved in RNA-DNA hybrids have important implications for human disease because mutations of these proteins have recently been linked to multiple neurological syndromes.
Telomere looping
Telomeres are protected from recognition by the DNA repair machinery and the DNA damage checkpoint machinery via the formation of a lariat structure, thereby ‘hiding’ the DNA end. Additionally, telomeres have been shown to form larger chromatin loops, which may be important for gene regulation and heterochromatin establishment. We have recently developed a 3C (chromatin conformation capture)-based approach to detect the loop structure. This system allows us to explore how telomere looping is regulated in terms of cell cycle, telomere length and genetic context.
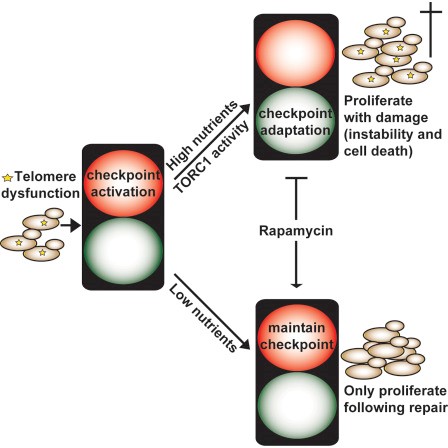
Checkpoint adaptation
We have shown that checkpoint adaptation can be inhibited by metabolic interventions such as rapamycin (TOR inhibitor) treatment or caloric restriction. As chemotherapeutics also induce chronic DNA damage, we are currently investigating whether similar metabolic interference could enhance chemotherapies and prevent the formation of “escaper” cells, that have adapted and therefore escaped the checkpoint. We are currently investigating the relationship between acquired drug resistance and checkpoint adaptation.
We are using a combination of the genetic model system budding yeast and human tissue culture to answer these fundamental questions related to DNA damage, checkpoint regulation and repair. The aim is that a better understanding of these basic questions will eventually translate into better interpretation of diseases such as the neurological Aicardi-Goutières syndrome or ALS (where RNA-DNA hybrid metabolism is defective) and help to render anti-cancer treatments more specific and lessen their side-effects.