Schick Lab
Research Publications Group Members BiographyChromatin regulation
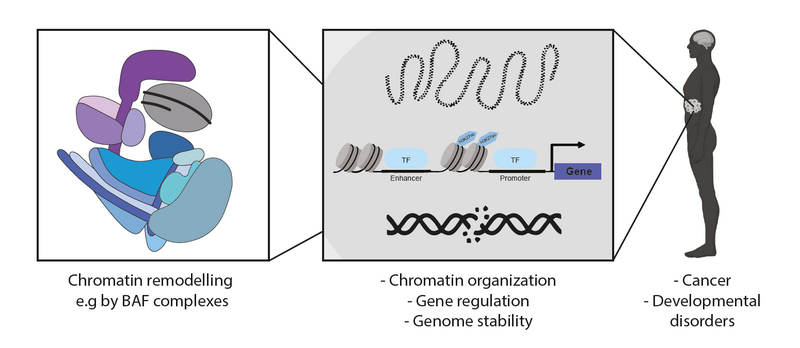
The packaging of genomic DNA into chromatin enables its incorporation into the nucleus and provides a complex platform for multi-layered genome regulation and maintenance. Chromatin modifying enzymes have evolved to keep chromatin flexible and adjustable in accordance with the requirements of the cell. Polymorphic ATP-dependent chromatin remodellers modulate chromatin composition and DNA accessibility and are thus essential regulators for all DNA-dependent processes such as transcription, DNA repair or replication. Given their importance, it is not surprising that their dysregulation, for example by mutations in genes encoding subunits of these complexes, is associated with developmental and age-related diseases and can affect lifespan.
The research in our lab aims to elucidate how chromatin-modifying events are regulated and integrated, as well as their impact on cellular processes in higher eukaryotes. Furthermore, we study the molecular basis of diseases caused by aberrant regulation of chromatin modifiers. We use a combination of genomics, proteomics, molecular biology, screening and imaging techniques to dissect the functions of these remodellers under normal, age- and disease-related conditions in mouse and human model systems. We also aim to elucidate the mechanisms of resilience and ultimately identify novel interventions that could be used for therapeutic purposes.
Chromatin regulation by BAF chromatin remodellers
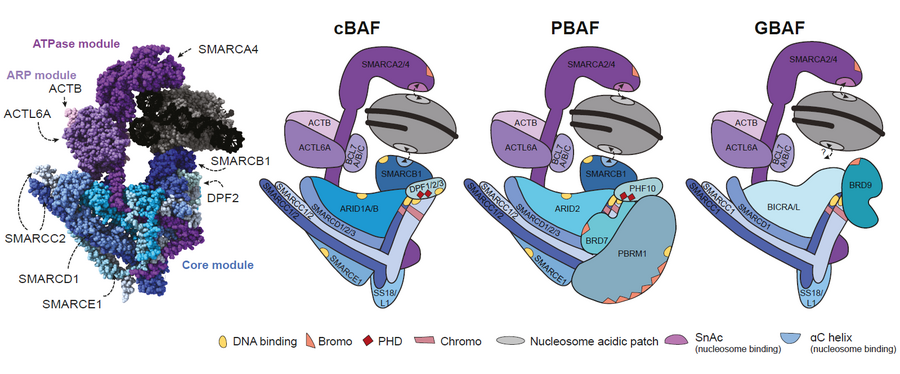
BRG1- or BRM-associated factor (BAF) complexes are adenosine triphosphate (ATP)-dependent chromatin remodelling enzymes that utilise energy from ATP hydrolysis to eject nucleosomes or slide them along the DNA. This allows parts of the genomic DNA to become either amenable or inaccessible to other factors such as transcription factors and DNA repair enzymes. Consequently, these chromatin remodellers are required for all cellular processes involving the genome.
BAF complexes are comprised of several subunits, which are encoded by more than 30 genes. These subunits assemble in a modular, combinatorial and sometimes cell type-dependent fashion into a large variety of different complexes. Their polymorphic occurrence is further enhanced by post-translational modifications and various transient interaction partners influencing their functions. Three main subgroups with partially distinct biological roles are currently recognised based on their specific incorporated subunits. However, our understanding of the molecular function and regulation of individual subcomplexes and how they integrate with other regulatory mechanisms is still incomplete.
Chromatin remodelling in development and disease
BAF complexes can exert highly specialised control of cellular processes through cell type-specific expression and composition patterns. For example, the composition of BAF complexes changes during neurogenesis; this is essential for differentiation and stage-specific gene expression programmes. The importance of BAF complexes is further highlighted by the fact that mutations in BAF subunits can cause (neuro)developmental diseases. Moreover, large-scale genomic sequencing studies have revealed a high frequency of mutations in genes encoding BAF complex subunits across numerous tumour types. Because many BAF complex mutations are associated with various human diseases, unravelling the precise molecular function of BAF complexes and their components will be important to identify disease-specific mechanisms that can be targeted for therapy.
Created using BioRender.com
To investigate these fundamental as well as pathogenic mechanisms, we combine model systems such as stem cell differentiation and organoids with state-of-the-art experimental and computational techniques, including CRISPR/Cas9 genome editing, targeted protein degradation, (single-cell) genomics, proteomics, imaging, as well as biochemical and molecular biology methods.
Recruiting
If you are interested in joining our team, please contact us via email. We welcome highly motivated experimental and computational scientists at all career stages.