A proof-reading mechanism for mRNA splicing
RESEARCH HIGHLIGHT
Mainz, 19 March 2020 – The research teams of Julian König at the Institute of Molecular Biology and Michael Sattler at the Institute of Structural Biology in Munich have discovered the molecular basis for how the splicing factor U2AF2 ensures that mRNAs are correctly spliced.
Splicing is a crucial step in the maturation of pre-mRNAs into mature mRNAs. It is also important for generating diversity in mRNAs (and hence proteins) from individual genes. For splicing to occur, the cell must be able to recognise the splice sites that flank exons, so that the spliceosome can be recruited to the correct place. Failure to recognise a splicing site or recruiting spliceosomes to the wrong place can lead to intron inclusion, exon skipping, or splicing in the middle of an exon/intron, which can in turn lead to disease.
U2AF2 is an essential splicing factor that plays a crucial role in splice site recognition. It binds to poly-pyrimidine tracts (Py-tracts), which are located immediately upstream of exons, and serves as the key point for spliceosome assembly. However, Py-tract sequences have widely varying sequences – some bind to U2AF2 strongly while others bind weakly. This diversity allows some exons to be constitutively and others to be alternatively spliced, such that different protein isoforms can be produced for specific tissues and functions. But with so many different binding sites, how does U2AF2 ensure correct and reliable splicing?
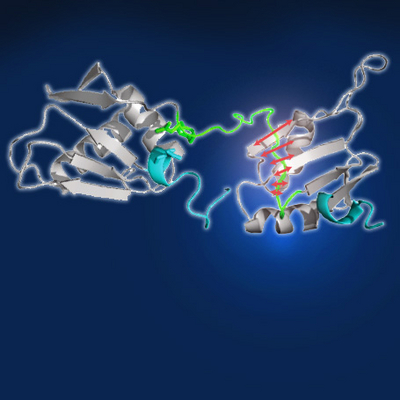
In their joint study published today in the Proceedings of the National Academy of Sciences USA (PNAS), Julian, Michael and their colleagues investigated this question by studying the 3D structure of U2AF2 using nuclear magnetic resonance (NMR). U2AF2 has two canonical RNA recognition motif domains, RRM1 and RRM2, which are connected by an intervening linker region. Surprisingly, they noticed that this linker region is actively involved in determining U2AF2’s RNA binding specificity. When the linker region was replaced, RRM1 and RRM2 binding increased more than four-fold at a weak Py-tract. This led them to speculate that the linker acts as a competitor that reduces RRM2 binding affinity at weak Py-tracts.
To test this, Julian’s team used a specialised technique called iCLIP to quantify RRM1 and RRM2 binding at hundreds of splice sites. When the linker region was present, RRM1/RRM2 bound strongly to strong Py-tracts, with decreased binding at medium and weak Py-tracts. In contrast, when they replaced the linker region with glycine and serine repeats, RRM1/RRM2 binding increased at both weak and strong Py-tracts. The mutant RRM1/RRM2 also erroneously bound to new sites, creating a dispersed binding pattern, and caused skipping of constitutive exons when overexpressed in human cell lines.
These results show that the linker region plays a vital role in allowing U2AF2 to discriminate between weak and strong Py-tracts, and offer a first insight into how splicing factors can maintain splicing fidelity despite binding to so many different sites. The first author of the study, Hyun-Seo Kang, says: “The linker region serves as a proof-reading mechanism that increases the binding specificity of U2AF2. This prevents U2AF2 from binding at cryptic splice sites and ensures that alternative and constitutive exons are correctly spliced to produce the right mRNA isoform.”
Further details Further information can be found at https://doi.org/10.1073/pnas.1913483117. Julian König is a Group Leader at the Institute of Molecular Biology in Mainz. Further information about research in the König lab can be found at www.imb.de/research/koenig.
About the Institute of Molecular Biology gGmbH The Institute of Molecular Biology gGmbH (IMB) is a centre of excellence in the life sciences that was established in 2011 on the campus of Johannes Gutenberg University Mainz (JGU). Research at IMB focuses on three cutting-edge areas: epigenetics, developmental biology, and genome stability. The institute is a prime example of successful collaboration between a private foundation and government: The Boehringer Ingelheim Foundation has committed 154 million euros to be disbursed from 2009 until 2027 to cover the operating costs of research at IMB. The State of Rhineland-Palatinate has provided approximately 50 million euros for the construction of a state-of-the-art building and is giving a further 52 million in core funding from 2020 until 2027. For more information about IMB, please visit: www.imb.de.
Boehringer Ingelheim Foundation The Boehringer Ingelheim Foundation is an independent, non-profit organization committed to the promotion of the medical, biological, chemical, and pharmaceutical sciences. It was established in 1977 by Hubertus Liebrecht (1931–1991), a member of the shareholder family of the company Boehringer Ingelheim. With the Perspectives Programme “Plus 3” and the Exploration Grants, the foundation supports independent junior group leaders. It also endows the internationally renowned Heinrich Wieland Prize as well as awards for up-and-coming scientists. In addition, the Foundation is donating a total of 154 million euros from 2009 to 2027 to the University of Mainz for the Institute of Molecular Biology (IMB). Since 2013, the Foundation has been providing a further 50 million euros for the development of the life sciences at the University of Mainz.
Press contact for further information :Dr Ralf Dahm, Director of Scientific Management, Institute of Molecular Biology gGmbH (IMB), Ackermannweg 4, 55128 Mainz, Germany. Phone: +49 (0) 6131 39 21455, Fax: +49 (0) 6131 39 21421, Email: press(at)imb.de